Aar-O-Vent®
Even minimal amounts of air and dirt in a hot water system can cause major issues affecting efficient operation, maintenance costs, and corrosion. The Thrush Aar-O-Vent® air and dirt removal device has been independently proven to remove:
- 100% of free air
- 100% of entrained air
- 99.7% of dissolved air
- 99% of dirt particles as small as 17 microns
As a result, your hot water systems can operate at peak velocity with virtually no corrosion or water flow issues.
customer testimonials
One of the reasons we began our relationship with Thrush was the Aar-O-Vent®. It is superior to competing products in the market.”
Tim Graydon, Vice President, Dowdy and Associates, Inc.
From Problem to Solution
Air and dirt in your hot water system could be causing significant problems
Such as:
- Decreased flow rate and distribution over the zone
- Reduced heat transfer resulting in labor-intensive manual bleeding of air
- Annoying “pinging” and “waterfall” noises
- Circulator cavitation and premature pump and heat exchanger failure
- Increased maintenance costs
- Energy waste
Removing air from your system can be a constant challenge due to undersized expansion tanks, system fills and refills, diffusion through non-metallic tubing, and ever-changing solubility. The best solution to prevent these air and water issues is with the Thrush patented Aar-O-Vent®, a high-performance air and dirt elimination device.
The Aar-O-Vent® “Ultimate Solution”
Only a high-performance air & dirt eliminator device with superior coalescing medium design, such as the Aar-O-Vent®, can remove and eliminate air down to a level that can actually absorb and remove air trapped in pockets and high points in the system. The Aar-O-Vent® is unique in that it has two driving forces to bring all three varieties of air out of solution.
- The design of the unit creates low-pressure regions to remove:
- 100% of free air (large visible bubbles)
- 100% of entrained air (air suspended in fluid in the form of small bubbles)
- 99.7% of dissolved air (air that is in solution in a fluid)
- The catalytic ability of the stainless-steel surface creates a very, very small electrical current that helps draw dissolved oxygen out of water.
At the same time, the Aar-O-Vent®’s coalescing removal separator media removes 99% of debris particles as small as 17 microns from circulating fluid that threatens to erode pump impellers, seals, and valve seats, along with piping and system components. The design of the Aar-O-Vent® forces dirt particles to the bottom sediment collection area and out of the flow path. This allows for easy removal through the blowdown valve without affecting system pressure.
The near-total air and dirt removal by Aar-O-Vent® stops corrosion within your system, plus eliminates heat transfer problems associated with trapped and entrained air, saving you money.
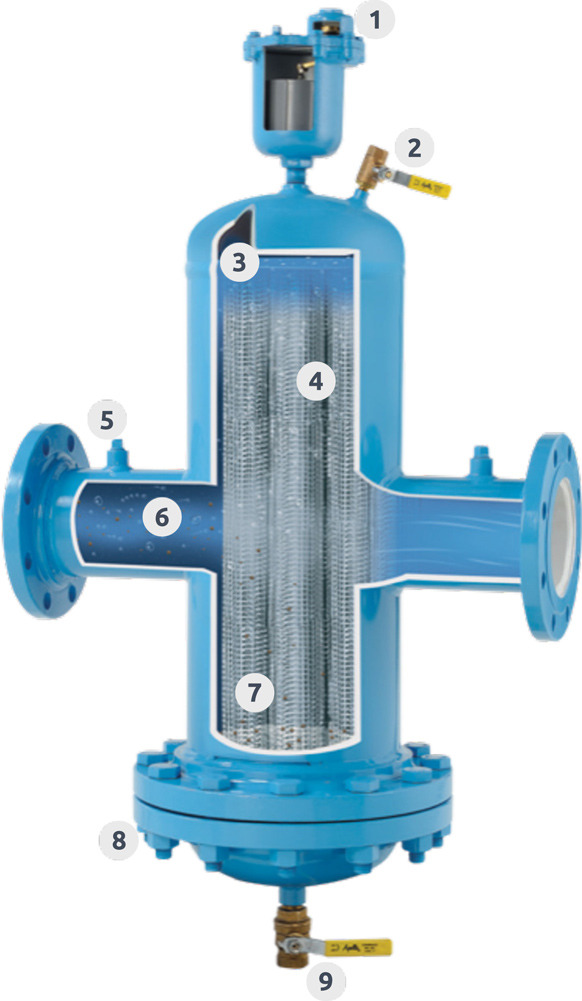
Aar-O-Vent® Anatomy
Here is a closer look at what makes the Aar-O-Vent® the best air and dirt separator in the industry with unmatched efficiency. Beyond the standard features of our models, Thrush offers customized options for your specific applications.
1. High Capacity Air Eliminator - The Thrush Model 720 High Capacity Air Eliminator releases air as fast as it can be separated. The 720 will not allow air back into the system, even if a vacuum occurs.
2. Skim Valve - Floating debris can be flushed out by opening the skim valve.
3. Air Bubbles - Large air bubbles quickly rise to the top of the vessel and into the vent. Micro bubbles coalesce and form larger bubbles. Entrained air is pulled out of solution and forms micro bubbles.
4. Stainless Steel Construction - The coalescing medium separates the air and dirt from the water. Stainless steel construction provides durability and long life.
5. Gauge Taps - Gauge taps on both inlet and outlet connections.
6. System Water - The system water contains air bubbles, entrained air, and dirt particles.
7. Filtration - Dirt particles are forced from the water and collect in the bottom of the vessel.
8. Removable Bottom Cover - Should the need to clean the coalescing medium arise, the standard removable bottom cover provides ease of removal and cleaning.
9. Blow Down Valve - Collected sediment can be flushed out through the blow down valve.
Catalytic Effect
Stainless steel is a reasonable and cost-effective metal that provides a good degree of catalytic ability. In fancy talk that means that it lowers the activation energy for a chemical process to take place which, in this case, is dissolved air coming out of the water (AKA coming out of solution), like carbon dioxide bubbles come out of pop (but for a different reason) once opened.
There are other metals that provide more significant catalytic properties, such as platinum which is used in a car’s catalytic converter for example. But these other alloys / exotic metals cost significantly more and are often costlier to work with.
In this sense, typical stainless steels of 304 & 316 provide a good cost balance between performance, durability, and cost. Stainless steel provides a better catalytic ability than carbon steel and copper and has the advantage of being more resistance to rust or other oxidation on its surface. Carbon steel rusts making its surface not effective and copper develops a green oxidation film on it further reducing its ability to provide catalytic ability.
If you have ever put your tongue on stainless steel, it produces a very small tingle effect, that is a very small electrical current that gets generated on the surface of the metal in question. It is this ability of stainless steels and other similar metals to provide a very small electrical current on their surface that helps to draw the dissolved oxygen out of solution (water).
The Aar-O-Vent®, in ADDITION to the catalytic property of stainless steel, ALSO draws the air out of solution by creating low-pressure areas/volumes. In these low-pressure regions, the dissolved air has an AFFINITY to come out of solution.
So, for the Aar-O-Vent®, you have two driving forces to bring the air out of solution:
a) catalytic surface of the stainless steel (this is a chemical effect)
b) the geometry of the unit (this creates low-pressure regions)
Independent Lab Test Results
Need a Custom Solution?
Our engineers can design custom Aar-O-Vent® systems to fit your precise application. All models are backed by our lifetime warranty.
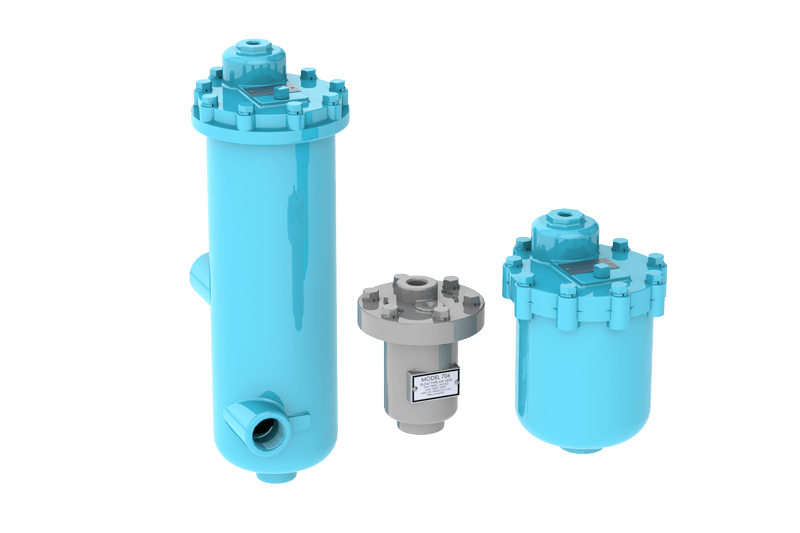
Thrush makes it easy for customers to find the right Aar-O-Vent® to meet their needs by providing options for air-only eliminators, dirt-only eliminators, and combination air and dirt eliminators. We stock air and dirt models for immediate delivery.

Aar-O-Vent® - Air Eliminator & Dirt Separator - Standard Velocity
With removable cover or fixed head cover
Product Details
Aar-O-Vent® - Air Eliminator & Dirt Separator - High Velocity
With removable cover or fixed head cover
Product Details
Aar-O-Vent® - Dirt & Sediment Separator - Standard Velocity
With removable cover or fixed head cover
Product Details
Aar-O-Vent® - Dirt & Sediment Separator - High Velocity
With removable cover or fixed head cover
Product Details


Aar-O-Vent® - Hydraulic Separator - Standard Velocity
With removable cover or fixed head cover
Product Details
promising quality since 1923
For All Your Hydronic Solutions, Think Thrush.
We don’t just talk about the quality and superiority of our products. We prove it. Every Thrush product is skillfully designed and rigorously tested to meet our standards. And our standard is simple: our heating and cooling products need to be the best in the Hydronics market.
Related Products
Ready to Start a Project?
Use our interactive Rep Locator Tool to Find a Thrush Co. Representative near you.
Find a Rep Near You